Understanding how organisms adapt to changing environments and how we can boost biodiversity is increasingly important as global warming continues to affect our planet. Dr Joana Meier (2018), the College’s William Bateson Fellow, spoke at the Beaufort Society Annual Meeting 2019 and shared new insights into evolution derived from her study of cichlid fish.
As a child I enjoyed nature and was fascinated by the huge diversity of animals and plants around us. I wondered how all of these species evolved. As a biology student, I was intrigued to find out that 160 years after the publication of On the Origin of Species we still have many open questions on this topic. For example, why are some animals or plants much faster at generating new species than others? This was my major question as a PhD student at University of Bern in Switzerland.
Sometimes many new species evolve in rapid succession, with each new species being adapted to a different way of living, such as eating something different. Biologists call this an ‘adaptive radiation’. For instance, when the ancestral Darwin’s finch colonised the Galapagos Islands, there were many different seed types that no bird was eating. No single species would be able to feed on all of these seed types as they require different beaks to crack them or get them out from their seed cases. Over time, different species evolved, each with a different beak well-adapted to eat a different kind of seed.

The fastest adaptive radiation occurred in cichlid fish in East Africa’s Lake Victoria. Here, 500 species evolved in just 15,000 years! This is extraordinarily fast, considering that speciation usually takes about a million years.
Professor Ole Seehausen at University of Bern in Switzerland has been studying these cichlid fish for over two decades. It was a great privilege to join his group as a PhD student to figure out how these fish were able to diversify so rapidly. Cichlids are beloved aquarium fish because many of them are very colourful. They occur in South America, Africa and India and in total encompass over 2,000 species, with most of these species occurring in the African Great Lakes. The 500 species in Lake Victoria live in different parts of the lake, from open water to muddy lake bottom or shallow rocky shores.
Cichlid fish also eat different things: some species eat insect larvae, others scrape algae off the rocks, crush snails or hunt zooplankton. Each species prefers a different part of the vast menu the lake offers. Some cichlid species are even adapted to feeding on scales of other fish or stealing their eggs.
I wanted to investigate how such vastly different species could have evolved so incredibly fast. The 500 species estimated to exist in Lake Victoria are all derived from the same ancestral lineage, which also formed adaptive radiations in multiple other lakes in the region.
In contrast, four other cichlid lineages also colonised these same lakes but none of them speciated even once. There was probably just not enough time for them to generate even a single new species. To figure out what is so special about the cichlid lineage that diversified so rapidly, I first had to figure out where it came from. Who are its ancestors? To answer this question, I went to Lake Victoria and caught lots of cichlid fish together with a fieldwork team. We identified the fish, photographed them and took tissue samples for DNA analyses.
My work shows that hybridisation can sometimes increase biodiversity by speeding up the process of speciation
In addition, I had access to samples from Ole’s huge cichlid collection and from collaborators. We ended up with about 240 cichlid samples including all cichlid lineages that might be closely related with these Lake Victoria cichlids and that might have been in contact in the past.
By analysing the DNA of these fish, I found out that the closest relatives of the Lake Victoria cichlids occur in the Congo drainage system. However, I also discovered a suspiciously high genetic similarity between Lake Victoria cichlids and more distantly related cichlids from the Upper Nile drainage. This can only be explained by hybridisation, ie interbreeding.
We found that the Lake Victoria cichlids are all of hybrid origin between Congolese and Nilotic cichlids. This hybrid ancestry likely also explains why they were able to generate so many species so rapidly. Usually, speciation takes very long because beneficial genetic changes are rare to occur. However, if two species combine their genes through interbreeding, their hybrid offspring are extremely diverse.
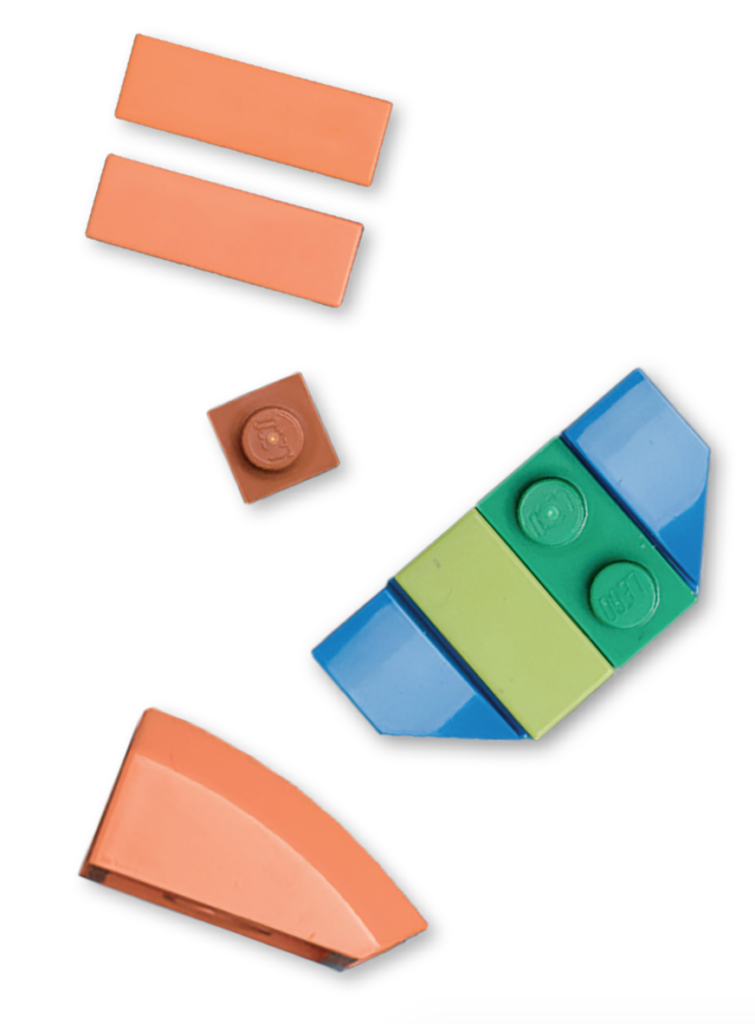
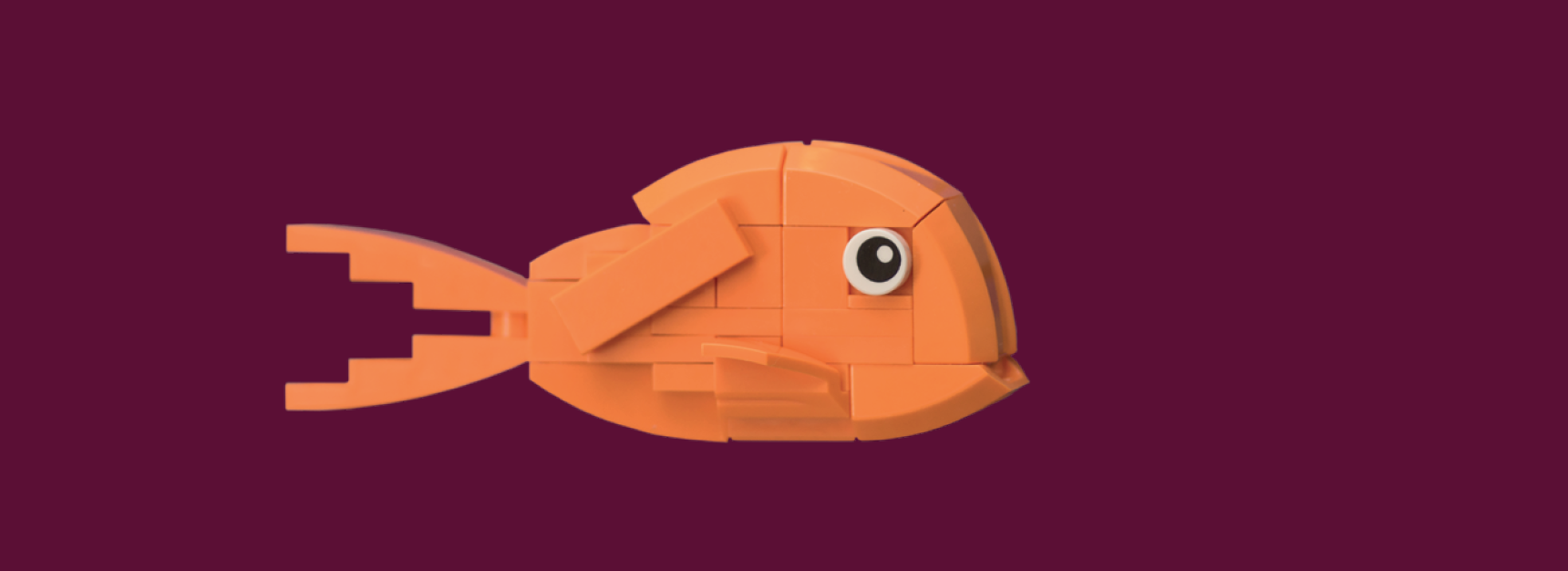
Some of these hybrids combine parental traits in a way that makes them better at feeding on certain things or living in specific conditions. This is easiest explained with a LEGO analogy: if you mix the LEGO tiles of a helicopter and a truck, you can easily rearrange them into many different vehicles with different functions. This is likely how these cichlids were able to generate new species so rapidly. They could quickly specialise on different food types as they already started with high genetic variation, whereby some hybrids were better at scraping algae and others were better at hunting little fish. They could circumvent the long waiting times for beneficial changes to occur by chance.
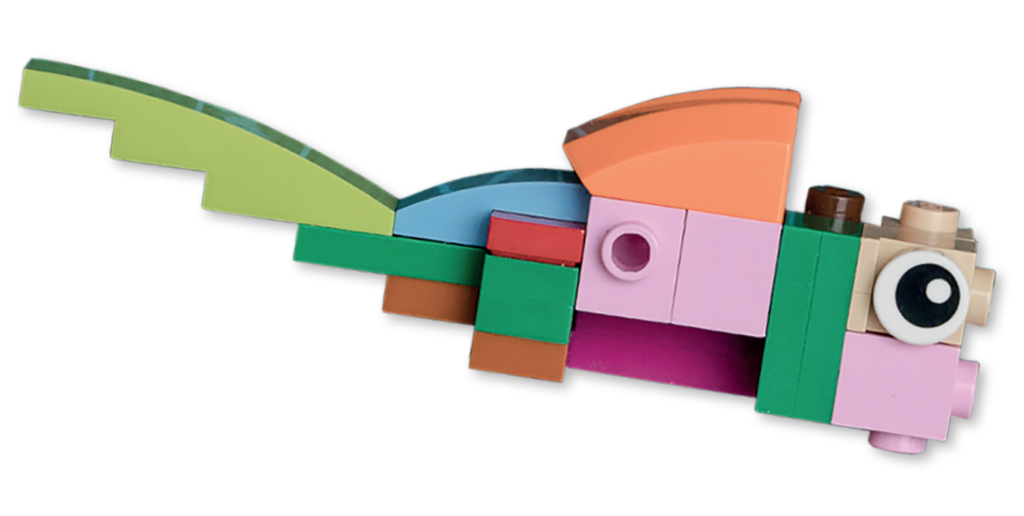
Hybridisation has traditionally been viewed as bad for biodiversity as it can lead to species fusion. However, my work shows that hybridisation can sometimes increase biodiversity by speeding up the process of speciation.
During my Research Fellowship at St John’s College, made possible by a generous bequest from the late Derek Austen (1964), I want to find out how generalisable this finding is. Did other animals and plants that speciated rapidly also interbreed in the past? Is hybridisation even a requirement for rapid speciation? I am currently testing these questions with South American butterflies.
Through collaborations, I also work on Hawaiian spiders and African daisies. If I find the same mechanisms to explain rapid evolution of new species in all of these different animals and plants, this would revolutionise our understanding of speciation.
Written by
As the William Bateson Fellow at St John’s, Joana is studying the evolutionary biology of cichlid fish and South American butterflies.